Viable air sampling USP 797 plays a pivotal role in the pharmaceutical industry, ensuring the sterility of manufacturing environments. It empowers quality control professionals with the knowledge and techniques to monitor and control microbial contamination, safeguarding the integrity of pharmaceutical products and patient safety.
This comprehensive guide delves into the intricacies of USP 797, providing a thorough understanding of its significance, methods, equipment, procedures, data analysis, and quality assurance measures. Embark on this journey to master the art of viable air sampling and elevate your pharmaceutical manufacturing practices.
USP 797 Background and Overview

USP 797 is a crucial standard that sets guidelines for ensuring the quality of compounded sterile preparations (CSPs) in healthcare settings. It plays a vital role in safeguarding patient safety by minimizing the risk of contamination and ensuring the efficacy of CSPs.
USP 797 applies to all healthcare facilities that prepare CSPs, including hospitals, pharmacies, and clinics. It provides comprehensive guidance on various aspects of CSP preparation, including personnel training, environmental monitoring, and quality control.
History and Revisions of USP 797
The first edition of USP 797 was published in 2004. Since then, it has undergone several revisions to keep pace with advancements in pharmaceutical compounding and to address emerging challenges in healthcare.
- USP 797 (2004): Established the foundation for CSP preparation standards.
- USP 797 (2008): Included guidance on personnel training and environmental monitoring.
- USP 797 (2019): Incorporated new technologies and updated requirements for sterility testing.
Viable Air Sampling Methods
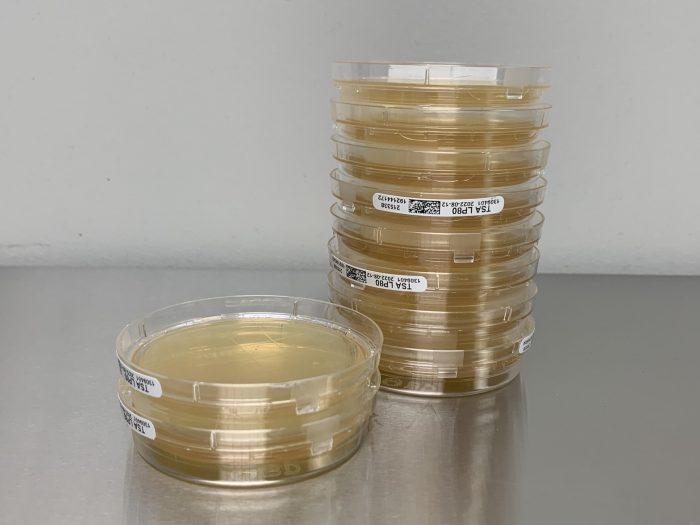
Viable air sampling methods are techniques used to collect and analyze microorganisms present in the air. USP 797 specifies several methods for viable air sampling, each with its advantages and disadvantages.
Impaction
Impaction methods rely on impinging air onto a solid surface, such as an agar plate or a membrane filter. The microorganisms in the air are physically captured on the surface, allowing for subsequent incubation and colony counting.
Viable air sampling according to USP 797 requires specific environmental conditions to ensure accurate results. Like Katherine who pays $43 a month , maintaining the right conditions for viable air sampling can be a significant expense. However, it’s essential to invest in these conditions to ensure the accuracy and reliability of the results.
- Advantages:Simple to use, inexpensive, and can collect both viable and non-viable particles.
- Disadvantages:Can be less efficient for collecting smaller particles, and the agar surface may dry out during sampling.
Filtration
Filtration methods use a filter to trap microorganisms from the air. The filter is then incubated, and the colonies that grow are counted.
- Advantages:Efficient for collecting both viable and non-viable particles, and can be used for large volumes of air.
- Disadvantages:Can be more expensive than impaction methods, and the filter may clog during sampling.
Centrifugation
Centrifugation methods use a rotating device to separate microorganisms from the air. The air is drawn into the device, and the microorganisms are centrifuged onto a collection surface.
- Advantages:Can collect both viable and non-viable particles, and can be used for large volumes of air.
- Disadvantages:More complex and expensive than impaction or filtration methods, and may not be suitable for all environments.
Factors to Consider
When selecting a viable air sampling method, several factors should be considered, including:
- The type of microorganisms being sampled
- The concentration of microorganisms in the air
- The volume of air to be sampled
- The desired accuracy and precision of the results
- The cost and availability of the equipment
Equipment and Materials

Viable air sampling necessitates a specific set of equipment and materials to ensure accurate and reliable results. These components play crucial roles in capturing and analyzing airborne microorganisms, providing valuable data for environmental monitoring and control.
Essential Equipment
- Air sampler:A device that actively draws air through a filter or growth medium to capture airborne microorganisms. Specifications include flow rate, sample volume, and filter type compatibility.
- Filters:Porous membranes or growth media that trap airborne microorganisms for subsequent analysis. Specifications include pore size, particle retention efficiency, and compatibility with the air sampler.
- Incubator:A controlled environment that provides optimal conditions for microbial growth and colony formation on the filter or growth medium. Specifications include temperature, humidity, and incubation time.
- Colony counter:A device used to enumerate the number of microbial colonies formed on the filter or growth medium. Specifications include magnification, lighting, and counting grid.
Calibration and Maintenance, Viable air sampling usp 797
Proper calibration and maintenance of equipment are essential to ensure accurate and reliable results. Air samplers should be calibrated regularly to maintain the desired flow rate and sample volume. Filters should be inspected for damage or contamination before use. Incubators should be monitored for temperature and humidity stability.
Colony counters should be calibrated to ensure accurate colony counting.
Additional Materials
- Sterile gloves:To prevent contamination during sample handling.
- Sterile forceps:To transfer filters or growth media.
- Culture media:To support microbial growth on filters or growth media.
li> Data collection and analysis software:To record and analyze sampling data.
Sampling Procedures: Viable Air Sampling Usp 797
Viable air sampling involves collecting airborne microorganisms onto a growth medium to assess the microbial contamination levels in a controlled environment. The sampling procedures are critical to ensure accurate and reliable results.
The following steps Artikel the general procedures for conducting viable air sampling:
- Prepare the sampling site:Ensure the sampling site is clean and free of potential contaminants. Avoid sampling near open doors, windows, or areas with high foot traffic.
- Calibrate the air sampler:Calibrate the air sampler according to the manufacturer’s instructions to ensure accurate flow rate and sampling time.
- Select the sampling method:Choose the appropriate sampling method based on the specific application and environment. Common methods include active air sampling (using an air sampler) and passive air sampling (using a settling plate).
- Collect the sample:Position the air sampler or settling plate at the desired sampling location and collect the sample for the specified duration.
- Handle and store the sample:Handle and store the sample properly to maintain its viability and prevent contamination. Follow the manufacturer’s instructions for sample storage and transportation.
Critical Parameters
Several critical parameters must be controlled during viable air sampling to ensure accurate and reliable results:
- Sampling time:The sampling time should be sufficient to collect a representative sample of the airborne microorganisms. The duration will vary depending on the environment and the expected contamination level.
- Flow rate:The flow rate of the air sampler should be calibrated and maintained to ensure the collection of a representative sample. The flow rate will determine the volume of air sampled and the number of microorganisms collected.
- Environmental conditions:Environmental conditions, such as temperature, humidity, and air movement, can affect the viability and distribution of airborne microorganisms. These conditions should be monitored and controlled during sampling to ensure accurate results.
Data Analysis and Interpretation
Data analysis and interpretation are crucial steps in viable air sampling, allowing researchers to make informed conclusions about the microbial contamination levels in a given environment. This involves employing various methods to analyze the collected data, perform calculations, and interpret the results.
Statistical Techniques
- Descriptive statistics:These provide a summary of the data, including measures like mean, median, mode, range, and standard deviation. They help understand the central tendency and variability of the data.
- Inferential statistics:These allow researchers to make inferences about the population from which the sample was drawn. Techniques like hypothesis testing and confidence intervals are used to determine the significance of differences between groups or to estimate population parameters.
Microbial Count Calculations
Microbial counts are calculated based on the volume of air sampled and the number of colonies observed on the growth medium. The formula used is:
CFU/m3= (Number of colonies / Volume of air sampled) x Dilution factor
The dilution factor accounts for any dilutions made during sample preparation or analysis.
Interpretation of Results
The interpretation of results involves comparing the microbial counts to established standards or guidelines. This helps determine the level of microbial contamination and its potential impact on the environment or personnel.
- Low levels:Typically indicate a well-controlled environment with minimal microbial contamination.
- Moderate levels:May suggest the need for increased monitoring or corrective actions to reduce contamination.
- High levels:Indicate significant microbial contamination and may pose a health risk or require immediate remediation.
Quality Assurance and Control

Quality assurance and control (QA/QC) measures are crucial for ensuring the accuracy and reliability of viable air sampling data. These measures help to minimize errors and ensure that the data collected is representative of the actual environment being sampled.
Training
Thorough training is essential for personnel involved in viable air sampling. Training should cover all aspects of the sampling process, including equipment operation, sampling techniques, and data interpretation.
Proficiency Testing
Proficiency testing involves the analysis of samples by an external laboratory to assess the accuracy and precision of the sampling and analysis methods. Regular proficiency testing helps to identify any potential biases or errors in the sampling process.
Equipment Validation
Equipment validation involves testing and verifying the performance of the air sampling equipment to ensure that it meets the specified requirements. This includes checking the accuracy of the flow rate, the efficiency of the filter, and the sensitivity of the detector.
Establishing and Maintaining a Quality Assurance Program
A comprehensive QA/QC program should be established and maintained to ensure the ongoing accuracy and reliability of viable air sampling data. This program should include:
- Written procedures for all aspects of the sampling process
- Regular calibration and maintenance of equipment
- Data review and validation
- Corrective action procedures for any deviations from the established protocols
General Inquiries
What is the purpose of USP 797?
USP 797 establishes standardized methods and procedures for viable air sampling in pharmaceutical manufacturing environments, ensuring the control and monitoring of microbial contamination.
What are the key components of USP 797?
USP 797 encompasses various aspects, including sampling methods, equipment specifications, sampling procedures, data analysis techniques, and quality assurance measures.
Why is viable air sampling important in pharmaceutical manufacturing?
Viable air sampling helps detect and quantify airborne microorganisms, enabling manufacturers to assess the effectiveness of their contamination control strategies and maintain sterile manufacturing conditions.