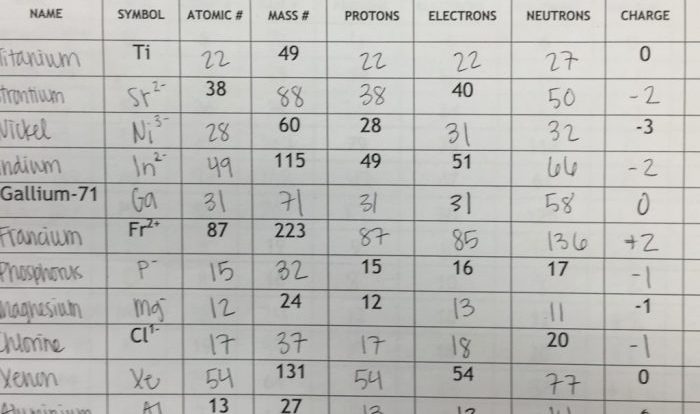
An hcl solution has a ph of 3.350 – An HCl solution with a pH of 3.350 presents a captivating subject, inviting exploration into its multifaceted nature. This solution exhibits unique properties and plays significant roles in various fields, making it a topic worthy of in-depth examination.
This discourse will delve into the concept of pH and its measurement techniques, unravel the chemical properties of HCl solutions, and explore their diverse applications. Furthermore, the safety considerations associated with HCl solutions and their environmental impact will be thoroughly addressed.
pH Measurement

pH is a measure of the acidity or basicity of a solution. It is defined as the negative logarithm of the hydrogen ion concentration in moles per liter (mol/L).
pH is a crucial parameter in chemistry, as it affects the behavior and properties of chemical reactions. It is used in various fields, including analytical chemistry, environmental science, and biochemistry.
Methods to Measure pH
- pH Meters:pH meters are electronic devices that measure pH directly. They consist of a pH electrode and a reference electrode, which are immersed in the solution.
- Indicators:Indicators are chemical substances that change color depending on the pH of the solution. They are used in simple pH measurements, such as litmus paper.
Factors Affecting pH Measurements
- Temperature:Temperature affects the pH of a solution. As temperature increases, the pH decreases.
- Electrode Calibration:pH electrodes need to be calibrated regularly to ensure accurate measurements.
Properties of HCl Solutions
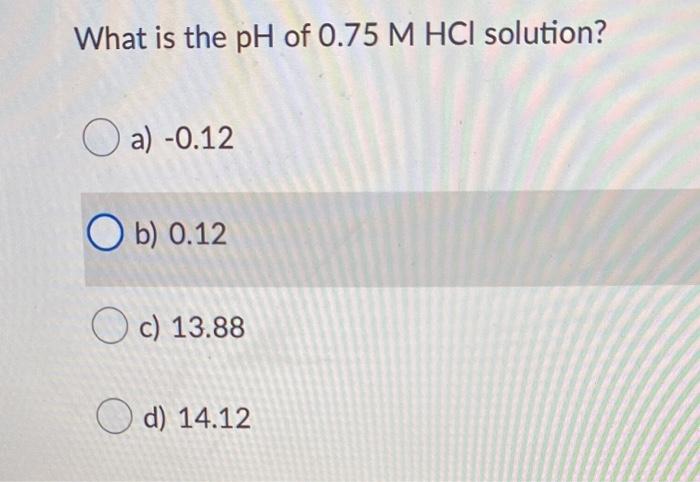
Hydrochloric acid (HCl) is a strong acid that dissociates completely in water, releasing hydrogen ions (H+) and chloride ions (Cl-).
Relationship between HCl Concentration and pH, An hcl solution has a ph of 3.350
The concentration of HCl in a solution is inversely proportional to its pH. As the HCl concentration increases, the pH decreases.
Corrosive Nature of HCl Solutions
HCl solutions are highly corrosive and can cause severe burns to skin and tissue. Proper safety precautions, such as wearing gloves and protective clothing, should be taken when handling HCl solutions.
Applications of HCl Solutions
HCl solutions have numerous applications in various industries.
Metalworking
- Pickling:HCl solutions are used to remove rust and scale from metal surfaces before further processing.
- Etching:HCl solutions are used to etch metal surfaces, creating designs or patterns.
Food Processing
- Acidulant:HCl solutions are used as an acidulant in food products, such as beverages and condiments.
- Preservative:HCl solutions can inhibit the growth of bacteria in food products.
Water Treatment
- Coagulation:HCl solutions are used to adjust the pH of water, promoting the coagulation of impurities.
- Ion Exchange:HCl solutions are used to regenerate ion exchange resins.
Neutralization Reactions: An Hcl Solution Has A Ph Of 3.350
Neutralization reactions involve the reaction of an acid with a base, resulting in the formation of a salt and water.
When HCl reacts with a base, such as NaOH, the following reaction occurs:
HCl + NaOH → NaCl + H2O
Applications of Neutralization Reactions
- Acid-Base Titrations:Neutralization reactions are used in acid-base titrations to determine the concentration of an unknown acid or base.
- Stomach Acid Neutralization:Antacids, such as sodium bicarbonate, neutralize stomach acid, providing relief from heartburn.
Key Questions Answered
What factors can affect pH measurements?
Temperature and electrode calibration are crucial factors that can influence pH measurements.
What safety precautions should be taken when handling HCl solutions?
Due to their corrosive nature, HCl solutions require proper handling, including the use of protective gear and proper ventilation.