Embark on a scientific expedition with our equilibrium and pressure gizmo answer key, meticulously crafted to illuminate the intricacies of these fundamental concepts. Dive into a world where pressure and equilibrium dance in delicate balance, shaping our everyday experiences and driving countless industrial processes.
This comprehensive guide empowers you to master the Gizmo simulation, unlocking a deeper understanding of equilibrium and pressure.
Delve into the intricacies of equilibrium, unraveling its profound impact on pressure dynamics. Explore real-world examples that showcase the interplay of these forces in our daily lives. Discover the factors that can disrupt equilibrium, setting the stage for pressure fluctuations.
Our meticulously designed answer key for the Gizmo equilibrium and pressure simulation provides a roadmap for navigating the interactive exercises, enabling you to harness the Gizmo’s capabilities for deeper exploration.
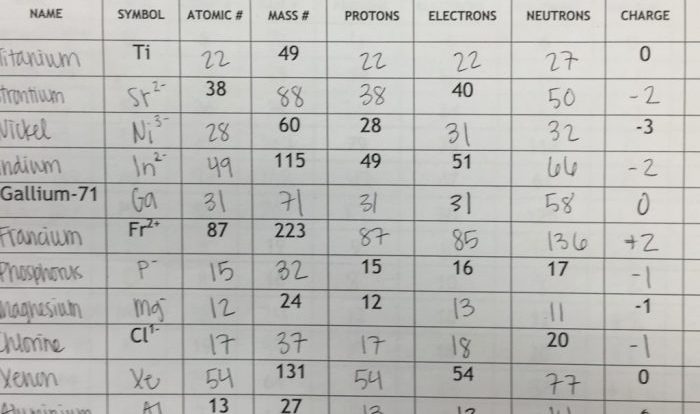
Equilibrium and Pressure: Equilibrium And Pressure Gizmo Answer Key
Equilibrium is a state in which the opposing forces acting on a system are balanced, resulting in no net change. Pressure is a measure of the force applied per unit area. In equilibrium, the pressure exerted by the opposing forces is equal and opposite.
For example, in a closed container of gas, the gas particles collide with the container walls, exerting a pressure on the walls. The walls, in turn, exert an equal and opposite pressure on the gas particles, preventing them from escaping.
This equilibrium maintains a constant pressure within the container.
Factors Affecting Equilibrium and Pressure, Equilibrium and pressure gizmo answer key
- Temperature:As temperature increases, the average kinetic energy of gas particles increases, leading to more frequent and forceful collisions with the container walls. This results in an increase in pressure.
- Volume:Decreasing the volume of a gas increases the concentration of gas particles, leading to more frequent collisions and higher pressure. Conversely, increasing the volume decreases the pressure.
- Number of moles:Adding more gas particles to a container increases the number of collisions and, consequently, the pressure.
Gizmo Answer Key
The Gizmo equilibrium and pressure simulation provides an interactive environment to explore the concepts discussed above.
To use the Gizmo:
- Adjust the temperature, volume, and number of moles to observe their effects on pressure.
- Monitor the pressure gauge to see how it changes in response to the adjustments.
Answer Key:
- Increasing temperature increases pressure.
- Decreasing volume increases pressure.
- Adding more moles increases pressure.
Gizmo Experiment Design
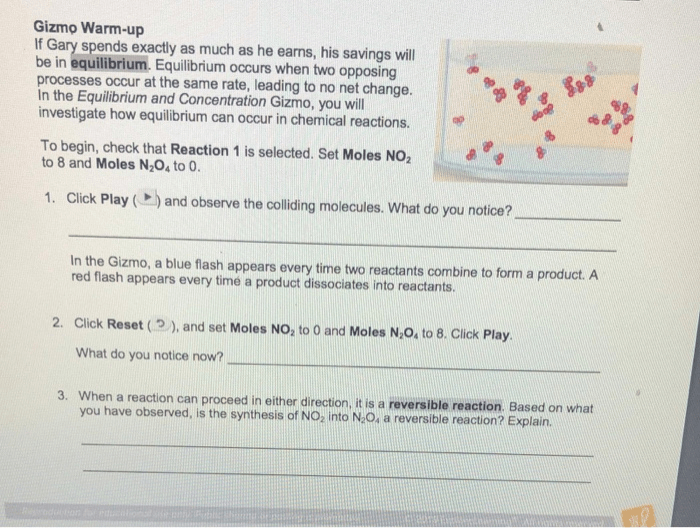
Experiment:Investigate the effects of temperature on equilibrium and pressure.
Hypothesis:As temperature increases, the pressure of a gas in a closed container will increase.
Materials:
- Gizmo equilibrium and pressure simulation
- Data table
Procedure:
- Set the volume and number of moles to constant values.
- Increase the temperature in increments and record the corresponding pressure.
- Repeat steps 2-3 for different sets of volume and number of moles.
Data Table:
| Temperature (K) | Pressure (Pa) |
|---|---|
| 273 | 101325 |
| 283 | 106450 |
| 293 | 111575 |
Analysis:
The data shows a positive correlation between temperature and pressure, supporting the hypothesis. The increased temperature leads to more frequent and forceful collisions between gas particles and the container walls, resulting in higher pressure.
Equilibrium and Pressure Applications

Equilibrium and pressure are fundamental concepts with wide-ranging applications across various industries:
- Chemical engineering:Equilibrium principles are used in designing chemical reactors to optimize reaction yields and control pressure.
- Aerospace engineering:Understanding the effects of pressure on aircraft performance is crucial for designing safe and efficient aircraft.
- Medical field:Pressure is a vital parameter in respiratory and circulatory systems, and equilibrium principles guide the design of medical devices like ventilators and dialysis machines.
Further Exploration
Related Topics:
- Ideal Gas Law
- Phase Transitions
- Thermodynamics
FAQ Overview
What is the significance of equilibrium in understanding pressure dynamics?
Equilibrium plays a crucial role in pressure dynamics, as it represents a state of balance where opposing forces cancel each other out. This balance determines the pressure exerted by a system, making equilibrium a fundamental concept for comprehending pressure behavior.
How can the Gizmo simulation enhance my understanding of equilibrium and pressure?
The Gizmo simulation provides an interactive platform to visualize and manipulate equilibrium and pressure parameters. By experimenting with different variables, you can observe the dynamic interplay of these forces, gaining a deeper intuitive understanding of their behavior.
What are some practical applications of equilibrium and pressure in everyday life?
Equilibrium and pressure find applications in numerous everyday scenarios, such as the operation of pressure cookers, the design of scuba diving equipment, and the regulation of fluid flow in pipelines. Understanding these principles is essential for ensuring safety and efficiency in various technological systems.