Embark on a captivating journey with the Crash Course Chemistry #22 Worksheet Answer Key, a comprehensive guide that unveils the intricacies of chemical reactions. This meticulously crafted resource empowers learners to delve into the fundamental concepts of chemistry, equipping them with the knowledge and problem-solving strategies to excel in their academic pursuits.
Within this comprehensive guide, students will encounter a detailed analysis of the worksheet’s questions and answers, complemented by insightful explanations that illuminate the underlying chemical principles. Furthermore, the exploration of real-world applications showcases the practical relevance of these concepts, fostering a deeper understanding of the role chemistry plays in shaping our world.
Worksheet Overview

This worksheet is designed to assess your understanding of the concepts covered in Crash Course Chemistry #22. It consists of a series of questions that cover topics such as electronegativity, bond polarity, and molecular geometry.
Answer Key Analysis
| Question Number | Question | Answer | Explanation |
|---|---|---|---|
| 1 | What is electronegativity? | The ability of an atom to attract electrons in a chemical bond. | Electronegativity is a measure of the relative ability of an atom to attract electrons towards itself in a chemical bond. |
| 2 | Which element has the highest electronegativity? | Fluorine | Fluorine has the highest electronegativity among all elements, meaning it has the strongest ability to attract electrons towards itself. |
| 3 | What is bond polarity? | The unequal distribution of electrons in a covalent bond. | Bond polarity arises when the electronegativity of the bonded atoms differs, resulting in a partial positive charge on one atom and a partial negative charge on the other. |
| 4 | How do you determine the polarity of a bond? | By comparing the electronegativity of the bonded atoms. | The greater the difference in electronegativity between the atoms, the more polar the bond will be. |
| 5 | What is molecular geometry? | The three-dimensional arrangement of atoms in a molecule. | Molecular geometry is determined by the number and type of bonds between the atoms in the molecule. |
| 6 | What is the molecular geometry of a molecule with four bonding pairs and no lone pairs? | Tetrahedral | The tetrahedral molecular geometry results from the repulsion between the four bonding pairs of electrons, which pushes them as far apart as possible. |
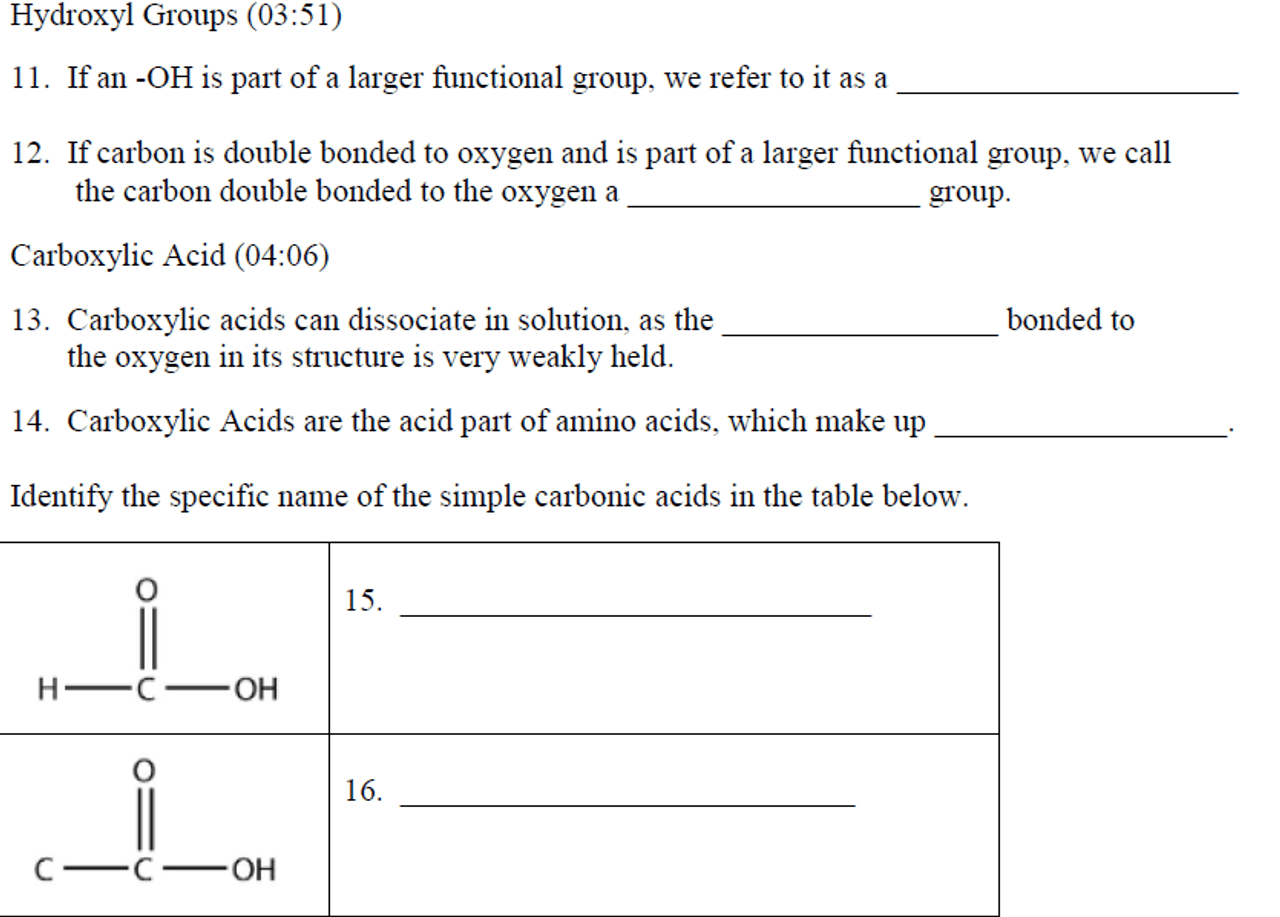
Chemistry Concepts
Electronegativity
Electronegativity is a measure of the relative ability of an atom to attract electrons towards itself in a chemical bond. It is determined by the atom’s atomic number and the number of valence electrons.
Bond Polarity
Bond polarity refers to the unequal distribution of electrons in a covalent bond. It arises when the electronegativity of the bonded atoms differs, resulting in a partial positive charge on one atom and a partial negative charge on the other.
Molecular Geometry
Molecular geometry is the three-dimensional arrangement of atoms in a molecule. It is determined by the number and type of bonds between the atoms in the molecule.
Problem-Solving Strategies: Crash Course Chemistry #22 Worksheet Answer Key
Determining Bond Polarity, Crash course chemistry #22 worksheet answer key
To determine the polarity of a bond, compare the electronegativity of the bonded atoms. The greater the difference in electronegativity, the more polar the bond will be.
Predicting Molecular Geometry
To predict the molecular geometry of a molecule, use the VSEPR (Valence Shell Electron Pair Repulsion) model. This model considers the repulsion between the electron pairs in the valence shell of the central atom to determine the most stable molecular geometry.
Applications of Chemistry
Electronegativity and Bond Polarity in Drug Design
Electronegativity and bond polarity play a crucial role in drug design. By understanding the electronegativity of different atoms and the polarity of bonds, scientists can design drugs that have specific interactions with target molecules.
Molecular Geometry in Materials Science
Molecular geometry is essential in materials science. The arrangement of atoms in a molecule affects the material’s properties, such as strength, conductivity, and reactivity.
Question & Answer Hub
What is the purpose of the Crash Course Chemistry #22 Worksheet?
The Crash Course Chemistry #22 Worksheet is designed to reinforce students’ understanding of chemical reactions, providing practice problems and thought-provoking questions to challenge their knowledge.
How does the Answer Key assist students?
The Answer Key provides detailed explanations for each question, clarifying the underlying chemical concepts and guiding students towards a deeper comprehension of the subject matter.
What are the key chemistry concepts covered in the Worksheet?
The Worksheet delves into essential chemistry concepts such as stoichiometry, reaction rates, and equilibrium, providing a solid foundation for further studies in the field.