What element has 26 electrons 29 neutrons and 26 protons – What element has 26 electrons, 29 neutrons, and 26 protons? This question delves into the fascinating world of atomic structure and the properties of elements. By exploring the unique characteristics of this element, we uncover insights into the fundamental building blocks of matter.
This element, with its specific arrangement of subatomic particles, exhibits distinct chemical and physical properties that shape its behavior in the world around us. Join us as we embark on a journey to identify this element and unravel its captivating story.
Element Identification
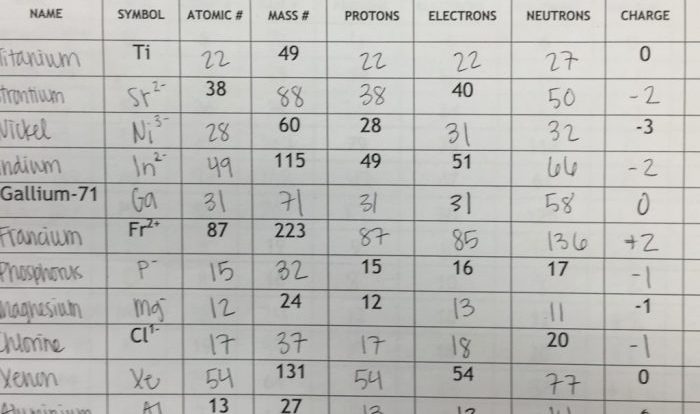
The element with 26 electrons, 29 neutrons, and 26 protons is iron, with the symbol Fe.
The atomic number of an element, which is the number of protons in its nucleus, defines its identity. In this case, the atomic number of 26 signifies that the element is iron.
Atomic Structure: What Element Has 26 Electrons 29 Neutrons And 26 Protons

Iron’s atomic structure consists of:
- 26 protons, located in the nucleus
- 29 neutrons, also located in the nucleus
- 26 electrons, arranged in orbitals around the nucleus
The protons and neutrons are tightly bound together in the nucleus, while the electrons occupy specific energy levels and orbitals.
Electron Configuration
The electron configuration of iron is [Ar] 3d 64s 2.
This indicates that iron has:
- 18 electrons in the core electron shells (the same as argon, represented by [Ar])
- 6 electrons in the 3d subshell
- 2 electrons in the 4s subshell
Chemical Properties
Iron is a transition metal, known for its reactivity and ability to form various chemical compounds.
- It readily undergoes oxidation, forming iron oxides such as Fe 2O 3(rust).
- Iron can exhibit multiple oxidation states, including +2 and +3.
- It forms coordination complexes with ligands, such as in hemoglobin, which binds oxygen in the blood.
- It has a high density of 7.87 g/cm 3.
- Its melting point is 1538°C (2800°F).
- Iron is a good conductor of heat and electricity.
- 54Fe (91.75% abundance)
- 56Fe (9.17% abundance)
- 57Fe (2.11% abundance)
Physical Properties

At room temperature, iron is a solid metal.
Isotopes
Iron has several stable isotopes, including:
Isotopes have the same number of protons and electrons, but different numbers of neutrons. They contribute to the element’s atomic mass and can affect its radioactive properties.
FAQ
What is the name and symbol of the element with 26 electrons, 29 neutrons, and 26 protons?
Iron (Fe)
How many protons does this element have?
26
What is the significance of the number of protons in an element?
The number of protons determines the element’s atomic number and defines its identity on the periodic table.